Citrate: Understanding Its Role and Importance in Chemistry
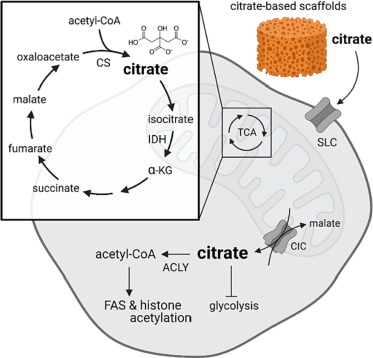
Citrate plays a crucial role in various biochemical processes, particularly in the energy metabolism of cells. As a key intermediate in the tricarboxylic acid (TCA) cycle, also known as the citric acid cycle, citrate is involved in generating adenosine triphosphate (ATP), which is essential for cellular energy. Understanding the importance of citrate and its derivatives, referred to collectively as citrates, sheds light on various metabolic pathways and their regulation within the body.
The significance of citrate extends beyond just being a metabolic intermediate. It acts as a metabolic regulator, influencing several key processes such as glycolysis and fatty acid synthesis. In this article, we will delve deeper into the chemical structure of citrate, its pivotal role in the TCA cycle, and its functions as a metabolic regulator. By analyzing these aspects, we can better appreciate the multifaceted nature of citrate in biochemistry.
- Overview of Citrate
- The Chemical Structure of Citrate
- The Role of Citrate in the TCA Cycle
- Citrate Synthase: The Catalytic Process
- Isomerization of Citrate: Implications and Functions
- Citrate as a Metabolic Regulator
- The Relationship Between Citrate and Glycolysis
- Citrate's Influence on Cellular Energy Control
- Conclusion: The Significance of Citrate in Biochemistry
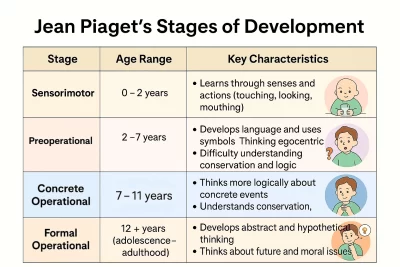
Overview of Citrate
Citrate is a tricarboxylic acid that plays a central role in cellular metabolism. It is formed from the condensation of oxaloacetate and acetyl-CoA in a reaction catalyzed by the enzyme citrate synthase. This reaction not only produces citrate but also releases coenzyme A, which is utilized in other metabolic pathways. Citrate can exist in several forms, termed citrates, depending on the pH and ionic conditions of the environment.
Citrate is not only important for energy production; it also has roles in various biochemical processes. This includes its participation in biosynthetic pathways and serving as a signaling molecule that can influence metabolic pathways. Given its fundamental nature in cellular processes, understanding citrate and how it functions is integral to the study of biochemistry.
The Chemical Structure of Citrate
The molecular formula for citrate is C6H5O7, and it consists of three carboxyl groups attached to a six-carbon backbone. Structurally, citrate can be represented in different forms, depending on whether it is in an anionic form or in equilibrium with its protonated counterparts. The presence of multiple carboxyl groups allows citrate to be highly soluble in water and play various roles in enzymatic reactions.
In its anionic form, citrate carries a negative charge, making it an excellent chelator. This property allows it to bind metals such as calcium, which is critical for several cellular processes, including muscle contraction and neurotransmitter release. By understanding the chemical structure of citrate, one can better comprehend its reactivity and the implications of its interactions within cellular pathways.
The Role of Citrate in the TCA Cycle
Citrate is the first product formed in the TCA cycle, highlighting its fundamental role in cellular respiration. The TCA cycle, also commonly referred to as the citric acid cycle, is a series of enzymatic reactions that lead to the oxidation of acetyl-CoA to produce ATP, NADH, and FADH2, which are critical for energy production. The pathway starts when citrate is formed from oxaloacetate and acetyl-CoA through the action of citrate synthase.
Once formed, citrate is then converted into a series of other intermediates through a series of reactions, ultimately regenerating oxaloacetate so the cycle can continue. The efficiency of this cycle is vital for ATP production, and thus, citrate's role as a TCA cycle intermediate is indispensable for maintaining cellular energy homeostasis.
Citrate Synthase: The Catalytic Process
Citrate synthase is the enzyme responsible for the synthesis of citrate from oxaloacetate and acetyl-CoA. This enzyme plays a crucial role in the TCA cycle, acting as a key regulatory step. The catalytic process involves the binding of oxaloacetate, followed by the addition of acetyl-CoA, which leads to the formation of a six-carbon intermediate that subsequently rearranges to form citrate.
This enzymatic reaction is highly regulated, particularly through the presence of substrates and feedback inhibition from products of the metabolic pathway. The effective modulation of citrate synthase activity ensures that the TCA cycle can respond appropriately to the energy needs of the cell, highlighting citrate's regulatory dynamics in metabolic flux.
Isomerization of Citrate: Implications and Functions
Once formed, citrate can undergo isomerization to yield isocitrate through the action of the enzyme aconitase. This transformation is an essential step within the TCA cycle, as isocitrate is further oxidized within the cycle to yield products that directly contribute to ATP synthesis. The isomerization of citrate underscores not only the flexibility of metabolic pathways but also the importance of citrate in shaping the flow of energy substrates through cellular respiration.
The ability of citrate to be converted to isocitrate and back to other forms demonstrates its dynamic role in metabolism. This isomerization does not just produce different substrates; it also affects the regulation of metabolic pathways and illustrates how citrate can serve as a biochemical hub, orchestrating various reactions essential for maintaining cellular mechanisms.
Citrate as a Metabolic Regulator
Citrate is recognized as an important metabolic regulator due to its ability to influence various pathways. High levels of citrate can inhibit key enzymes in glycolysis, such as phosphofructokinase, signifying a feedback mechanism that aligns with cellular energy needs. By serving as a signal of high energy status, citrate provides cellular feedback to adjust metabolic processes accordingly.
This regulatory function of citrate extends to lipid metabolism as well. Citrate plays a pivotal role in fatty acid synthesis by signaling the need for lipid storage when energy levels are abundant. This highlights citrate's function not just as an energy intermediate but as a key regulator orchestrating the balance between energy production and storage.
The Relationship Between Citrate and Glycolysis
The relationship between citrate and glycolysis is a prime example of how metabolic pathways are interconnected. Glycolysis, the process of converting glucose into pyruvate, is essential for generating precursors that fuel cellular respiration and produce ATP. When energy levels are sufficient, elevated citrate levels will inhibit enzymes in glycolysis , reducing the flow of substrates into this pathway and ensuring that the cell does not overproduce ATP.
This regulatory feedback ensures a balanced energy state within the cell, highlighting the importance of citrate not only as a metabolic intermediate but also as an essential signal in maintaining metabolic homeostasis. The inhibition of glycolysis by citrate parallels the effects driven by ATP and reflects the intricate balance cells must achieve to optimize their energy production processes.
Citrate's Influence on Cellular Energy Control
Citrate's influence extends to cellular energy control mechanisms beyond its direct metabolic roles. By modulating the activity of key enzymes and influencing key metabolic pathways, citrate ultimately affects the cells' ATP production capabilities. The balance between citrate synthesis, isomerization, and its roles as a regulatory molecule ensures that ATP synthesis is aligned with the cell's energetic needs.
As a metabolic signal, citrate can indicate the energy status of the cell, affecting metabolic processes such as gluconeogenesis and lipogenesis. This means that citrate not only serves as a building block for energy production but also acts as a key player in integrating various metabolic signals that inform the cell of its functional status.
Conclusion: The Significance of Citrate in Biochemistry
In conclusion, citrate is a fundamental compound with significant roles in cellular metabolism. Through its involvement in the TCA cycle, its structural characteristics, and its functions as a metabolic regulator, citrate stands out as a vital component of biochemical processes. Understanding citrate's multifaceted nature helps illuminate its importance in energy production, regulation, and cellular homeostasis.
The study of citrate and its relationships with other metabolic pathways continues to shed light on the complexities of biochemistry, reinforcing the significance of this compound in maintaining life processes. Further research into citrate's roles and its potential therapeutic applications could provide valuable insights into metabolic diseases and overall cellular function.
Did you find this article helpful? Citrate: Understanding Its Role and Importance in Chemistry See more here Education.
Leave a Reply




Related posts