Mercury Charge: Definition, Uses, Density, and Key Facts
Mercury, also known by its chemical symbol Hg, has fascinated scientists and alchemists for centuries. Often referred to as mercury charge, this unique element stands out for being the only metallic substance that is liquid at room temperature. In this article, we will explore the definition, properties, density, and a range of uses for mercury, encompassing its significance in both historical and contemporary contexts.
Despite its many applications, the health and environmental concerns associated with mercury are increasingly important in today’s world. As we delve deeper into the discussion of hg charge, we will uncover the various aspects of this element, including its common uses in medicine and industry, as well as the ongoing challenges regarding its toxicity and sourcing practices. Ultimately, a comprehensive understanding of mercury will aid in recognizing both its value and risks.
Definition of Mercury
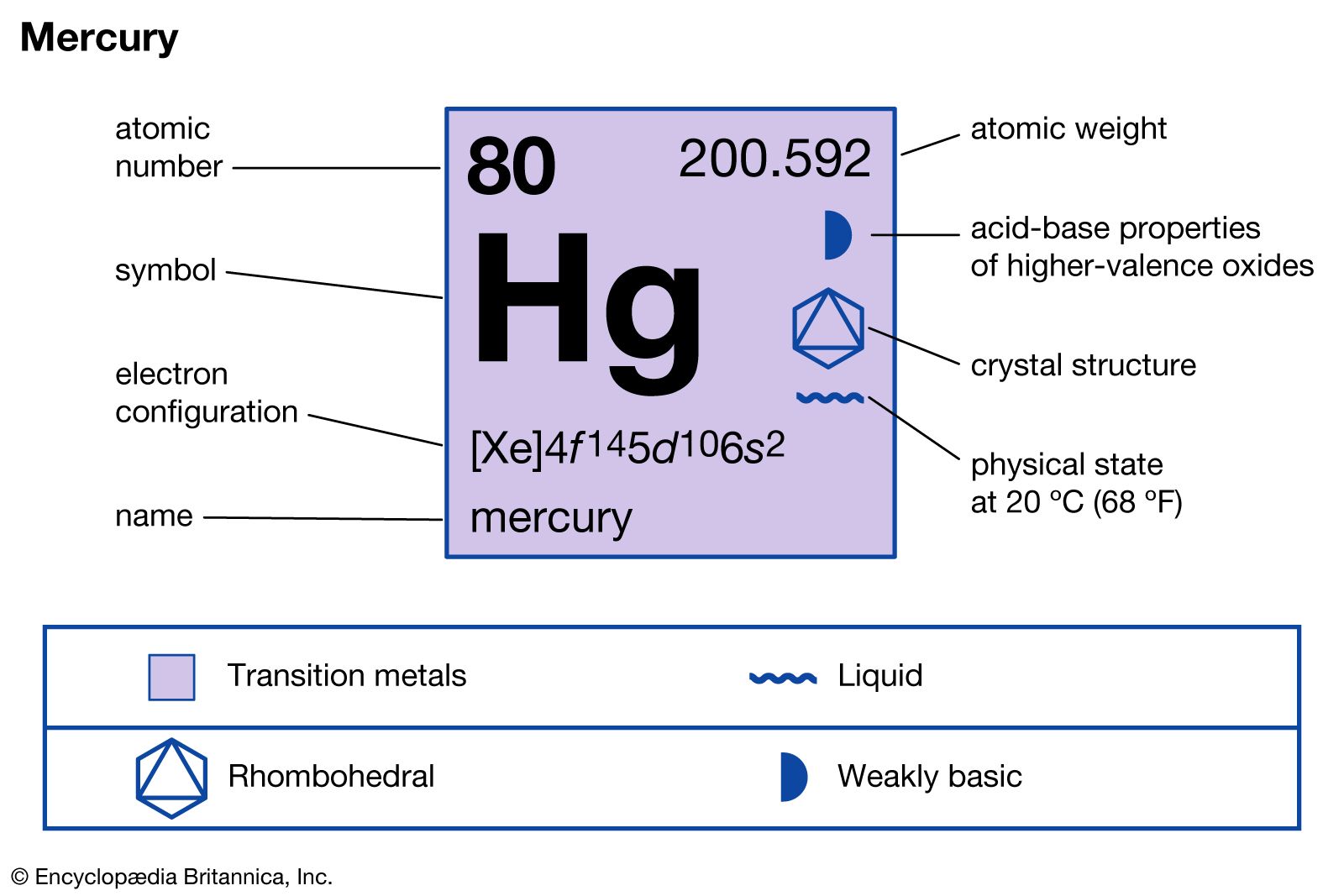
Mercury is a chemical element represented by the symbol Hg and atomic number 80. It belongs to the mercury group of the periodic table, specifically classified under the transition metals. This unique element has been well-documented in alchemical texts for centuries, showcasing its long-standing presence in human history.
Characterized by its high density and ability to form amalgams with a wide range of metals, mercury was historically used in electronic devices, thermometers, and various industrial applications. Its liquid state at room temperature, coupled with its ability to conduct electricity, further emphasizes the importance of understanding the charge of mercury and its various aspects.
Properties of Mercury
The properties of mercury are quite distinct, setting it apart from other metallic elements. It has a melting point of -38.83°C and a boiling point of 356.73°C, regulating its unique status as a liquid metal. The density of mercury is notably high, measuring approximately 13.6 grams per cubic centimeter, which explains its wide usage in applications requiring efficient weight measurements.
Aside from its physical properties, the mercuric charge is notable. Mercury commonly exhibits a charge of +1 and +2, depending on the oxidation state in various compounds. Understanding the charge of Hg is crucial for applications in chemistry and industry.
Density of Mercury
The density of mercury is one of its most remarkable features, making it an ideal candidate for applications where high mass is needed in a small volume. Its ability to remain liquid at room temperature allows for the use of mercury in devices that measure pressure, temperature, and volume accurately.
At around 13.6 g/cm³, the density of mercury poses a significant contrast to common liquids, such as water, which has a density of 1 g/cm³. This exceptional density makes mercury a preferred choice in many scientific instruments, such as barometers and manometers, providing precise measurements in a compact form factor.
Common Uses of Mercury
Mercury has been utilized across various industries due to its unique properties. Some of the common uses include:
- Thermometers and Barometers: Due to its predictable thermal expansion and high density, mercury serves as a reliable liquid in traditional thermometers and barometers.
- Dental Amalgams: Mercury is used to create amalgams with silver and other metals for dental fillings due to its ability to bond well with various materials.
- Industrial Applications: It is used in certain electrochemical processes, although its use is declining due to toxicity concerns.
Mercury in Medicine
Historically, mercury was widely used in medicine, notably in the treatment of syphilis and as a diuretic. These uses have largely been phased out in favor of safer alternatives as the toxicity of mercury became more widely recognized. Nonetheless, understanding the historical context of mercury charge opens discussions about medicinal chemistry and the evolution of treatment practices.
Mercury in Industry
In industrial contexts, mercury plays a role in various applications, including its use in fluorescent lamps, battery manufacturing, and as a catalyst in chlorine production. However, advances in technology have led to a decline in the demand for mercury in these sectors due to stricter environmental regulations and awareness of health risks.
Health and Environmental Concerns
One of the most pressing issues related to mercury is its toxicity. Exposure to this element can have significant effects on human health, potentially leading to neurological damage and other severe health issues. The Hg charges, especially in the form of methylmercury, are particularly harmful as they can accumulate in seafood, posing risks to health when these fish are consumed.
The environmental impact of mercury extraction and use is extensive, as it can lead to contamination of water supplies and biological organisms. Consequently, there has been a shift towards more environmentally friendly practices in the extraction and use of mercury to mitigate these risks.
Mercury Extraction and Sourcing
The primary source of mercury is the ore cinnabar (HgS), from which it is extracted through various methods. Historically, mercury mining occurred in several locations worldwide, but over 90% of the global supply now comes from China. As extraction continues, organizations and researchers are working on improving methods to make mercury sourcing less harmful to the environment.
Isotopes of Mercury
Mercury has seven stable isotopes, which include Hg-196, Hg-198, and Hg-200, among others. The isotopically pure mercury-198 is often used in scientific applications, particularly in nuclear medicine for imaging and radiation therapy. Understanding the isotopes and their properties contributes to a comprehensive view of the element and its many facets.
Conclusion
In understanding mercury — its charge, properties, uses, and the concerns surrounding it — we gain valuable insights into both its historical importance and current relevance. Despite its toxic nature, mercury remains a critical subject of study within chemistry and environmental science.
As regulations evolve and scientific advancements improve our understanding, the focus on safe handling and alternatives to mercury will likely continue. Emphasizing safer methods in both the extraction and application of mercury will play an essential role in protecting human health and our environment.
Did you find this article helpful? Mercury Charge: Definition, Uses, Density, and Key Facts See more here Education.
Leave a Reply




Related posts