
What are lutetium's uses, properties, and radioactivity
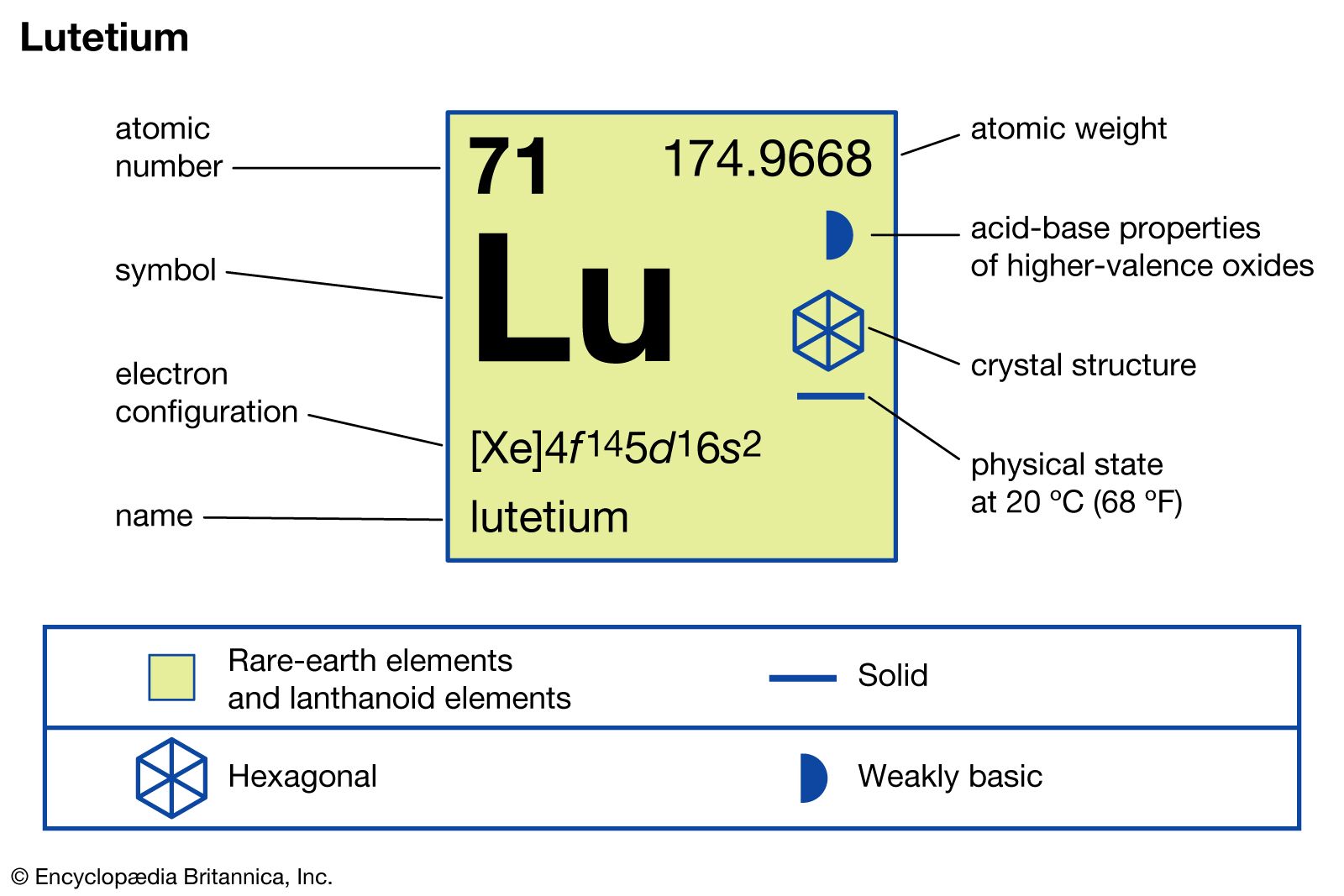
Lutetium, represented by the lu chemical element symbol Lu, is a unique lutetium element that has attracted attention for its diverse range of applications and intriguing properties. As the densest and highest-melting element of the lanthanide series in the periodic table, lutetium plays a significant role in both scientific research and industrial applications. Understanding what is lutetium used for can shed light on its importance in modern technology and science.
This article will explore the various aspects of lutetium, including its physical and chemical properties, its discovery and historical significance, and its sources in nature. We will also delve into its isotopes and radioactivity, particularly focusing on whether is lutetium radioactive and its implications. Finally, we will discuss the extraction methods, applications, and uses of lutetium element in the fields of research and industrial materials, especially in scintillators and optical lenses.
Overview of Lutetium
Lutetium (Lu) is classified as a rare-earth metal and belongs to the lanthanide series of the periodic table. It has an atomic number of 71 and is primarily known for its physical attributes, being a silvery-white metal that exhibits remarkable durability and stability in various environments.
The lu element was first isolated in the late 19th century, but its purity and applications were further explored in the early 20th century. It is an element that is not widely found in nature due to its rarity, often extracted from other minerals that contain rare-earth elements. The significance of lutetium in the scientific community has grown with advancements in technology, making it a critical part of various modern applications.
Physical and Chemical Properties
The physical and chemical properties of lutetium metal make it an essential element in many applications. With a melting point of over 1,540 degrees Celsius, it is the highest among all lanthanides, allowing it to maintain stability even under extreme temperatures. Lutetium has a density of 9.84 g/cm³, which is considered very high, contributing to its robust nature.
In terms of reactivity, lutetium is relatively stable in air, forming a layer of oxide that protects it from further oxidation. It can react with diluted acids to form lutetium salts but is notably unreactive with hydrofluoric acid, due to the formation of a protective layer. Additionally, lutetium exhibits paramagnetism at room temperature, which transitions to superconductivity at lower temperatures, making it valuable in specialized applications.
Discovery and Historical Significance
The discovery of lutetium is attributed to two chemists: Carl Auer von Welsbach and Georges Urbain, who independently isolated the element between 1907 and 1908. The discovery of the element Lu was significant in the context of solid-state physics and materials science, as it opened new avenues for research and development, especially in the domain of rare-earth metals.
The naming of lutetium is derived from Lutetia, the Latin name for Paris, reflecting its discovery's geographic significance. Since its identification, lutetium has been studied extensively, leading to a growing understanding of its properties and potential applications across various scientific fields.
Sources and Occurrence in Nature
Lutetium is not found in large quantities in nature, which adds to its allure and value. It primarily occurs in rare-earth minerals, often associated with other lanthanides. Monazite and bastnasite are among the minerals that contain lutetium, albeit in relatively low concentrations. The extraction process involves separating lutetium from other rare-earth elements, which can be challenging due to their similar chemical properties.
Trace amounts of lutetium can also be found in other commercially important minerals. Its distribution in the earth’s crust is approximately 0.5 parts per million, making it one of the lesser abundant rare-earth elements. Despite its rarity, advancements in mining and extraction techniques have made it feasible to obtain sufficient quantities of this metal for various applications.
Isotopes of Lutetium
Lutetium has several isotopes, with lutetium-175 being the most stable and abundant, constituting approximately 97.41% of natural lutetium. It possesses a half-life greater than the age of the Earth, making it significant for various scientific applications.
The other noteworthy isotope is lutetium-176, which is radioactive and has a half-life of around 37.7 billion years. This long half-life makes it particularly useful in geological dating, including dating meteorites and assessing the age of the Earth. The presence of these isotopes enhances the understanding of both the element’s stability and its potential uses in scientific research.
Radioactivity and Its Implications
When examining the radioactivity of lutetium, the focus is primarily on isotopes such as lutetium-176. Despite being a part of the lanthanide series, only certain isotopes exhibit radioactivity. The question is lutetium radioactive can be answered affirmatively, particularly concerning its radioisotopes, which are of interest in nuclear science and technology.
The radioactivity of lutetium and its isotopes has significant implications, particularly in the field of geology and planetary science, providing invaluable tools for dating and understanding the evolutionary history of planetary bodies. Furthermore, the study of its radioactive properties may enable advancements in the field of nuclear medicine and imaging, expanding the applicability of lutetium in various domains.
Extraction and Purification Methods
The extraction of lutetium involves several sophisticated techniques due to its scarcity in nature. The primary methods used include liquid-liquid extraction and ion-exchange techniques, which allow for the efficient separation of lutetium from other rare-earth elements.
Liquid-liquid extraction utilizes solvents to selectively dissolve specific elements while leaving others in a solid form, enhancing the purity of the extracted lutetium metal. On the other hand, ion-exchange processes involve the use of resin materials that interact with the ions in solution to separate lutetium from other elements. These advancements in extraction methods are crucial for obtaining high-purity lutetium, which is essential for its various applications.
Applications of Lutetium
The applications of the lutetium element are numerous and diverse, extending from scientific research to advanced technology. One of the primary uses of lutetium is in the development of high-quality scintillators, which are materials that display illumination when exposed to radiation. This property is harnessed in various fields, including medical imaging and radiation detection.
Another significant application of lutetium is in cutting-edge optical lenses. Given lutetium’s unique optical properties, it is increasingly employed in specialized optical materials that enhance the performance of lenses in various high-tech devices.
Lutetium in Research
Lutetium serves as an essential material in scientific research, where its unique properties are utilized in a myriad of experimental setups. Research activities often explore its magnetic and superconducting properties, contributing to advancements in condensed matter physics.
Furthermore, the integration of lutetium in nanotechnology research facilitates the development of novel materials with enhanced performance. The study of lutetium also leads to the discovery of new compounds with potential applications in catalysis and other chemical processes.
Use in Scintillators and Optical Lenses
The utilization of lutetium in scintillators has revolutionized certain fields, particularly in health and safety applications. The ability of lutetium to emit light in response to ionizing radiation makes it an indispensable component in the development of high-performance scintillation detectors.
Additionally, its unique optical characteristics make lutetium an important material for the fabrication of specialized optical lenses. Enhanced refractive properties allow for better performance in various optical devices, improving imaging resolution and quality.
Conclusion
lutetium is a rare-earth metal that possesses a range of intriguing physical and chemical properties. Its discovery marked a significant milestone in the study of lanthanide elements, and it continues to be of great importance in various scientific and industrial applications. From its unique isotopes used in dating meteorites to its critical role in developing scintillators and optical lenses, lutetium remains at the forefront of innovation and research.
As we explore the uses of lutetium element further, we gain a deeper understanding of its potential in a rapidly advancing technological landscape. With ongoing research and exploration, lutetium is poised to play an even more significant role in addressing various scientific challenges in the future.
Did you find this article helpful? What are lutetium's uses, properties, and radioactivity See more here Education.
Leave a Reply



Related posts