
What Are Line Spectra and Their Quantum Effects Explained
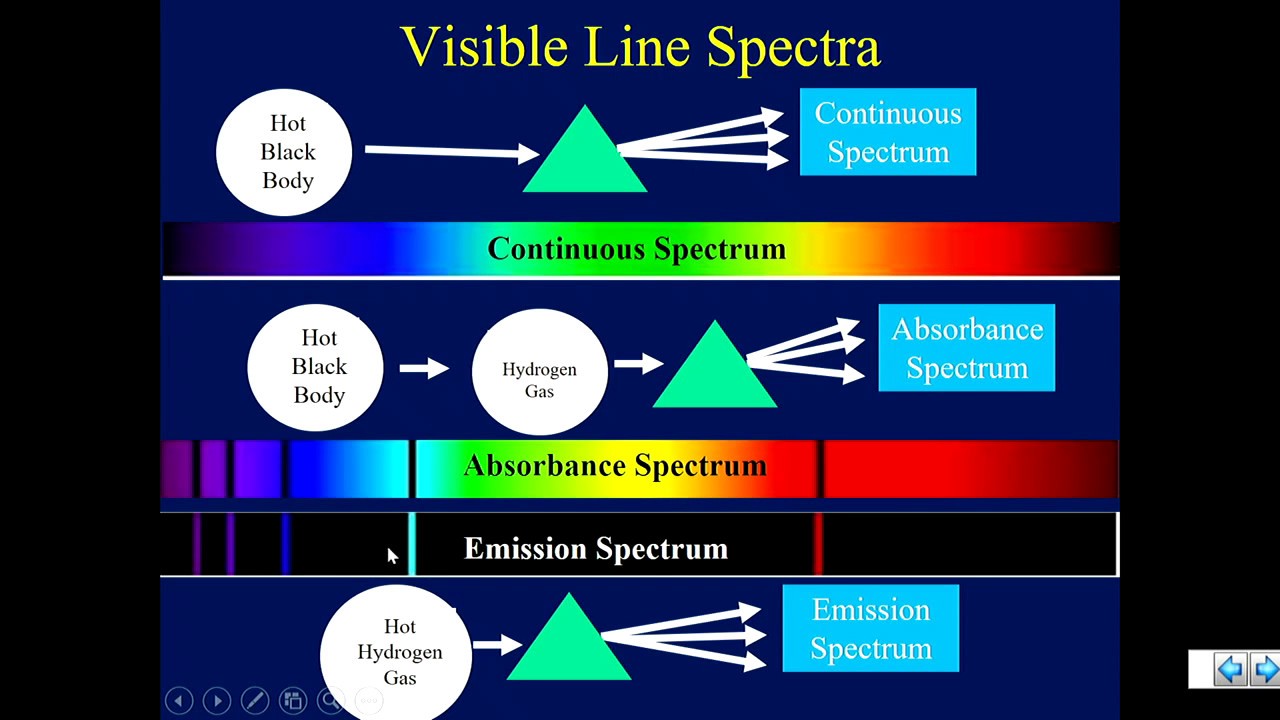
A line spectrum is a crucial concept in the field of spectroscopy, essential for understanding various quantum phenomena. By analyzing these spectrum lines, scientists can determine the composition and behavior of elements within different substances. The unique patterns formed by the line spectra of individual elements serve as fingerprints, revealing their presence and abundance in an array of materials.
As we delve deeper into the understanding of the spectrum quantum mechanics that govern these emissions, it becomes evident that the interactions between atoms and light play a fundamental role. The study of line spectra not only provides insights into atomic structure but also highlights the quantum effects that define the emission and absorption characteristics of light. By exploring these concepts, we can appreciate the intricate relationship between atoms, light, and the universe.
Understanding Line Spectra
In physics and chemistry, a line spectrum refers to a unique set of specific wavelengths emitted or absorbed by atoms or molecules. This phenomenon occurs when an electron transitions between quantized energy levels within an atom, leading to the release or absorption of energy in the form of light. Each element's energy transitions create a specific pattern of spectrum lines, making their line spectra distinctive.
The Nature of Light and Spectroscopy
To comprehend line spectra, it is essential first to understand the nature of light itself. Light behaves as both a wave and a particle, a duality that has significant implications on how spectra are formed. When light passes through a prism or a diffraction grating, it disperses into a spectrum, revealing its constituent wavelengths. This technique of analyzing light is known as spectroscopy, a powerful tool in both laboratory and astrophysical settings.
In line spectra, only specific wavelengths are observed, corresponding to discrete energy transitions within atoms. This differentiates them from continuous spectra, which consist of a smooth gradient of colors. Spectrum lines in line spectra appear as vertical lines on a graph, representing emitted or absorbed wavelengths. The technique of observing these line spectra has myriad applications, from identifying elemental composition to probing the physical conditions of stars.
Atomic Structure and Electron Transitions
The atomic structure is fundamental to explaining line spectra. Every atom consists of a nucleus surrounded by electrons in quantized energy levels, or shells. When an electron transitions between these energy levels, they either absorb or emit energy in the form of photons. The amount of energy released or absorbed corresponds to the difference between the two energy states, resulting in the formation of spectrum lines unique to each element.
For instance, when hydrogen atoms absorb energy, electrons can jump to a higher energy level. When they return to a lower state, they emit energy as a photon with a specific wavelength, creating a characteristic line spectrum. These unique patterns allow scientists to analyze and identify substances by examining their line spectra.
The Formation of Line Spectra
The process leading to the formation of line spectra involves both energy absorption and emission. When light is directed at an element, electrons may absorb energy and transition to higher states. Conversely, when electrons return to lower energy levels, they emit photons, which congregate at specific wavelengths, forming distinct spectrum lines.
Moreover, the linear spectrum displays these emitted spectrum lines clearly separated from one another. Each line corresponds to a specific transition within the atom, making these spectra particularly useful for distinguishing between elements. By studying the precise emission wavelengths, scientists can infer a material's composition and other critical physical properties.
Distinctive Characteristics of Atomic Spectra
The most intriguing feature of atomic line spectra is their uniqueness to each element, akin to a fingerprint. The energy level differences, which dictate the emitted wavelengths during electron transitions, are intrinsic to an element's atomic structure. Consequently, the quantum spectrum reflects the specific properties of each atom.
Another notable characteristic is the presence of certain lines being more pronounced than others. This variation depends on the population of atoms in different energy states and their probability of transitioning between these states. Thus, a line spectrum comprises bright lines against a dark background, representing specific wavelengths emitted by the atoms within a substance.
Applications of Line Spectra in Science
The applications of line spectra are vast and significant across multiple scientific domains. In chemistry, they serve as an analytical tool for identifying elements in a sample using techniques like flame tests or atomic absorption spectroscopy. As each element has its unique line spectrum, scientists can identify materials with remarkable accuracy.
In astrophysics, line spectra provide crucial insight into the universe's composition. By examining the spectrum lines emitted by distant stars, scientists can deduce their chemical makeup, temperature, density, and motion. This is because the absorption and emission features in the starlight are a direct consequence of interactions with the stellar atmosphere, revealing much about celestial phenomena.
- Identifying elements through flame tests and atomic absorption spectroscopy
- Analyzing stars and galaxies through their emission spectra
- Studying planetary atmospheres
- Applications in medical diagnostics
- Utilization in lasers and optical technologies
Quantum Effects Underlying Line Spectra
The underlying quantum effects that dictate the formation of line spectra stem from the principles of quantum mechanics. The discrete nature of energy levels within an atom reflects fundamental principles, such as quantization. When considering the interactions between electrons and photons, it becomes evident that each transition emits a photon of a specific energy, frequency, and corresponding wavelength.
An integral factor contributing to the occurrence of line spectra lies in the concept of quantized energy states. Electrons can only exist in specific energy levels and transition between them under certain conditions. This quantization leads to well-defined spectrum lines, reinforcing the signature that defines a particular element's emission or absorption characteristics.
Conclusion: The Significance of Line Spectra
In conclusion, line spectra are a profound manifestation of the interaction between light and atomic structure. The unique quantum spectrum lines produced by individual elements provide critical insights into both terrestrial and celestial phenomena. Through spectroscopy, we gain a better understanding of the composition of materials and the fundamental laws governing atomic behavior.
By appreciating the vast applications and implications of line spectra, we can acknowledge their importance in fields ranging from chemistry to astrophysics. As we continue to explore the universe's wonders, spectrum lines will remain an essential tool in our scientific arsenal, unveiling the mysteries that exist both around us and in distant reaches of the cosmos.
Did you find this article helpful? What Are Line Spectra and Their Quantum Effects Explained See more here Education.
Leave a Reply




Related posts