
What Is the Absolute Temperature Scale Used In Science
The absolute temperature scale is a crucial concept in the realms of thermodynamics and physical science. It allows scientists to express temperatures in a way that corresponds directly to the energy state of particles. At the heart of this scale lies the concept of absolute zero, the theoretical point at which a system's entropy reaches its minimum, resulting in a state of zero kinetic energy. This scale provides a common framework for understanding temperature, making it essential for various scientific calculations.
One of the most prominent scales used in this system is the Kelvin scale, which is defined as starting at absolute zero, aligning perfectly with the theoretical aspects of energy states. The significance of the Kelvin scale extends beyond mere measurement, as it influences fundamental laws of physics, including thermodynamics. The true temperature of a system, as defined by the absolute temperature scale, facilitates a more profound understanding of thermal processes and other scientific phenomena.
- Understanding Absolute Zero
- The Kelvin Scale: Definition and Significance
- Historical Context: The Evolution of the Kelvin Scale
- The Role of the Boltzmann Constant
- Comparing the Kelvin and Rankine Scales
- Practical Applications of Absolute Temperature in Science
- Conclusion: The Importance of Absolute Temperature in Thermodynamics
Understanding Absolute Zero
Absolute zero is defined as 0 K, equivalent to -273.15°C or -459.67°F. It represents the point at which the motion of atoms theoretically ceases, implying that no additional thermal energy can be extracted from a system. This concept is not just a theoretical abstraction; it has significant implications for the behavior of materials at extremely low temperatures. For instance, at temperatures approaching absolute zero, matter exhibits quantum properties that are not observable at higher temperatures.
The significance of absolute zero in the absolute temperature scale cannot be overstated. It serves as the reference point from which all thermodynamic calculations are derived. As we approach absolute zero, the motion of particles slows significantly. This provides invaluable insights into phenomena such as superconductivity, where materials exhibit zero electrical resistance at low temperatures—demonstrating the practical applications of understanding true temperature.
The Kelvin Scale: Definition and Significance
The Kelvin scale is the primary absolute temperature scale used in the scientific community. Defined by the International System of Units (SI), it is expressed in kelvins (K) and begins at absolute zero. The Kelvin scale is essential because it allows scientists and engineers to calculate energy transfers effectively without confusion caused by negative temperature readings, a problem encountered in other temperature scales.
One kelvin is equivalent to one degree Celsius; however, the two scales differ in their starting points. The Kelvin scale begins at 0 K and is directly proportional to the energy present in a system. Thus, for scientists, measuring temperature in kelvins allows for a direct interpretation of thermal energy, facilitating calculations in various scientific fields such as physics, chemistry, and engineering.
Historical Context: The Evolution of the Kelvin Scale
The development of the Kelvin scale is deeply rooted in the history of thermodynamics and the understanding of heat. In the mid-19th century, Lord Kelvin (William Thomson) proposed using absolute zero as a reference point, which significantly advanced the field of thermodynamics. His work established a clearer understanding of the relationship between temperature and energy, paving the way for the formal definitions we use today.
Before the acceptance of the absolute temperature scale, various other definitions and scales existed for measuring temperature, often leading to confusion and discrepancies in scientific communications. The transition to the Kelvin scale, defined based on fundamental constants such as the Boltzmann constant, helped standardize measurements and reduce errors. This evolution underscores the importance of a consistent and logical approach to scientific measurement, aligning more closely with the concept of true temperature.
The Role of the Boltzmann Constant
The Boltzmann constant is a fundamental physical constant that plays a crucial role in defining the Kelvin scale. It provides a bridge between macroscopic and microscopic properties of matter, linking the average kinetic energy of particles in a gas with the temperature of that gas. The constant is approximately 1.38 x 10^-23 J/K, and its significance extends beyond definitions; it aids in understanding how temperature affects state changes, chemical reactions, and other thermodynamic processes.
Through the application of the Boltzmann constant, scientists can derive equations that govern the behavior of systems at various temperatures. For example, the link between thermal energy and temperature allows researchers to calculate energies in gas equations, facilitating predictions about material behavior under different thermal conditions. In this way, the Boltzmann constant emphasizes the direct correlation between the absolute temperature of a system and its particle behavior, solidifying the connection between the macroscopic and the microscopic worlds.
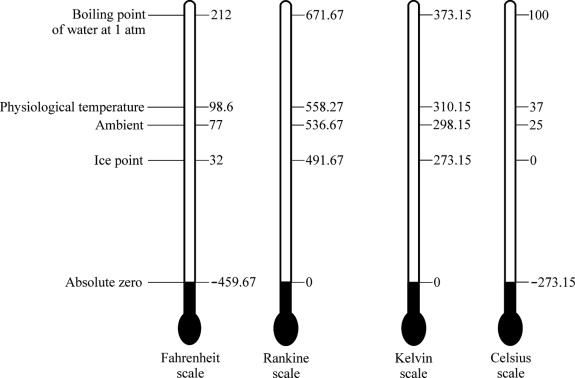
Comparing the Kelvin and Rankine Scales
While the **Kelvin scale** is predominantly used in most scientific fields, the Rankine scale is another absolute temperature scale, primarily utilized in engineering, particularly in thermodynamics in the United States. The Rankine scale is based on the Fahrenheit scale, with absolute zero defined as 0 °R, equivalent to 0 K or -459.67 °F. The freezing and boiling points of water are marked at 491.67 °R and 671.67 °R respectively.
One of the key differences between the Kelvin and Rankine scales is their increments: the Kelvin scale increments by degrees Kelvin, while the Rankine scale increments by degrees Rankine, which are equivalent to Fahrenheit degrees. Therefore, if one is converted from Kelvin to Rankine, for every degree increase in Kelvin, the corresponding increase in the Rankine scale is also one degree, but starts at a higher numerical value due to the different zero points.
This differentiation is pivotal when considering the application of thermodynamic equations, and it is essential for engineers who work with energy systems to select the appropriate temperature scale for their calculations, ensuring accurate interpretations of the true temperature across varying conditions.
Practical Applications of Absolute Temperature in Science
The implications of using the **absolute temperature scale**, particularly the Kelvin scale, extend far beyond theoretical exercises; it serves as a foundation for numerous practical applications in science and engineering. One significant area of application is in thermodynamics, where equations governing heat transfer, work, and energy conversions rely on temperature measurements expressed in absolute terms.
Additionally, the concept of absolute temperature is central to the study of material properties. For instance, materials may exhibit varying characteristics such as conductivity or resistance depending on their temperature; understanding these relationships provides insights into how materials can be manipulated for use in various technologies. In areas like semiconductor physics, where temperature shifts can lead to significant changes in material performance, applying absolute temperatures allows for reliable, repeatable results.
Moreover, in cryogenics—the study of materials at extremely low temperatures—scientists rely on the absolute temperature scale to explore phenomena such as superfluidity and superconductivity. Here, the precise measurement of true temperature plays a critical role in advancing our understanding of quantum mechanics and potential practical technologies such as loss-less electrical transmission.
Conclusion: The Importance of Absolute Temperature in Thermodynamics
The absolute temperature scale, particularly defined by the Kelvin scale, is a cornerstone of modern science. It provides an essential framework for understanding fundamental concepts in thermodynamics and facilitates scientific progress across various fields. The significance of absolute zero as the starting point allows scientists to comprehend energy states clearly and to create consistent laws that govern physical and chemical processes.
By utilizing this consistent scale, scientists are better equipped to derive meaningful conclusions from their experiments, leading to advances in technology and our understanding of the universe. The persistent reliance on the concept of true temperature ensures that research remains precise and aligns with the universal laws that govern the natural world, emphasizing the continued importance of the absolute temperature scale in scientific inquiry and application.
Did you find this article helpful? What Is the Absolute Temperature Scale Used In Science See more here General.
Leave a Reply






Related posts